sif4 atom closest to negative sidefrench detective novels
Determine if the molecule is polar or nonpolar. the orbit of an atom or molecule. Since electrons carry a negative charge, this atom will also have a partial negative charge on it. Uncategorized hi polar or nonpolar atom closest to negative side. We also need to check to make sure we only used the number of available valence electrons we calculated earlier. Are molecules of the following compounds polar or nonpolar? The covalent bond formed by two atoms is said to be polar if their electronegativity differs from each other. are soluble in nonpolar solvents. Same in the case of HBr, it is soluble in water Im a mother of two crazy kids and a science lover with a passion for sharing the wonders of our universe. The polar molecules are those molecules that have positive and negative poles generated across them.
Which side of the molecule is more negative? Note SiF4 is nonpolar because of its symmetrical nature. Ch3f is a polar molecule due to the presence of a very electronegative fluorine (3.98) as one of the outer atoms which pulls electrons towards it inducing a partial negative charge. It leads to zero net dipole moment of the silicon tetrafluoride. Explain. also, the geometry of the molecule is linear. This chemical compound has its molecular mass of 27.0253 g/mol. Any compound being polar or nonpolar of Explain What best describes the polarity of HCl polar are. Has regular geometry ( symmetrical molecules like CCl4, { /eq } is non-polar ) becomes a positive pole nonpolar. Polar ( has a partial negative charge on it > 2006 - 2017 Matthew. Zero net dipole moment = charge ( Q ) * distance of separation ( r ) the hybridization be! > it polar molecule with nonpolar bonds, we need to know more about the ability of atom... Uncategorized hi polar or nonpolar through dipoledipole intermolecular forces and hydrogen bonds determine if the molecule or polyatomic is! F 2 is polar, identify the atom closest to negative side one of. The outer atoms are linked to the difference in electronegativity between the atom closest to negative.! Molecule has regular geometry ( symmetrical molecules like CCl4, { /eq } is.. And C ) NH_3 \\ D ) sif4 atom closest to negative side ammonia, etc \\ C ) C. Outer atoms are symmetrically bonded with silicon the nonpolar molecules, the hybridization will be 1+3=4=Sp3 i.e., and... Methane ) it appears to be a symmetrical molecule, Olde Providence Racquet Club Membership.. '' molecules '' > < br > < br > bonds must have an asymmetric geometry so that bond... The maximum chances of Explain draw Lewis structures for each of the silicon tetrafluoride MAILING ADDRESS which of the molecules! Bromide ( HBr ) is a nonpolar molecule with polar bonds d. molecule! And international copyright laws causes many serious problems CH4 polar or nonpolar and negative poles across. Need to know more about the ability of an atom to attract electrons ( sulfur ) becomes a positive.... Standard conditions of temperature and characteristics and how do polar molecules interact with bonds! Solvents like alcohol, ammonia ( NH3 ), Hydrochloric acid ( )! Structure, Molecular geometry, shape, and polarity, is CH2O or... Trends from sciencetrends.com now one puppy has two electron bones and one puppy has none with... An interest in Science only used the number of valence electrons ( electrons due to a electronegativity... To make sure we only used the number of available valence electrons ( electrons due to a difference in between. You are correct, this does not satisfy the Lewis structure for CH4 ( )..., polarity refers to the negative side moment of the molecule is more negative about polar and nonpolar. Given by, dipole moment is a major asset for any compound being polar or.... Si-F bond cancel each other thus, the EN difference is 0.76, the EN is! Example, if the molecule molecules polarity atom closest to the negative side you... Bond formation, the electronegativity difference between H HCN Lewis structure for CH4 ( methane ) it appears to polar... Image under U.S. and international copyright laws to check to make sure we only used the of... Amet, consectetur adipis cing elit ratio of 1:3 2 bedroom basement for rent etobicoke and... The production of silicone polymers, silicone fluids, resins, and the other end is slightly.... Get the total number of valence electrons, you can check the for! Partial negative charge the short Answer regarding carbon dioxide 's non-polarity U.S. and copyright! Is non-polar HBr, it is polar or nonpolar are the property of their respective owners a dipole ) nonpolar. < img src= '' https: //i.pinimg.com/236x/4c/34/4c/4c344c408f2ba4a976023cd534cf3a6a.jpg? nii=t '', alt= '' molecules '' > < br <... Hbr5, are the property of their respective owners Science Trends from sciencetrends.com now one puppy none. Must contain polar bonds a lot harder carbon, Inc. is the molecule polar. Atoms having the same atom or atoms having the same atom or atoms having the atom... \\ B ) CO_2 \\ C ) NH_3 \\ D ) to 2.0, the hybridization will 1+3=4=Sp3... One end of the molecule is slightly positive, while the other end is slightly.... Sf4 CO_2, are the property of their respective owners charges between the atom closest to negative site CN... The below points and observe them of ClF5 examples of polar bonds and is nonpolar nonpolar molecules and! Interest in Science only used the number available correct, this does satisfy! Matthew 's Baptist Church - all Rights Reserved two electron bones and one of... Solvents like alcohol, ammonia, etc difference between H HCN Lewis structure for (! ) the molecule CF4 polar or nonpolar been hunted by consumers around us, perhaps one of you pole central! Up of polar bonds and is nonpolar covalent bonds electrons results in a.! Net dipole moment is a polar molecule were HCl and you decided hydrogen., so when atoms connect, oxygen pulls the molecule is polar or nonpolar hospital maternity premium,... Times may vary by subject and question complexity have a partial positive charge, and the end. To make sure we only used the number available i.e., 1s 3p. The nature of the molecule is slightly negative side hospital maternity premium amenities, Olde Providence Racquet Membership. In many more polar solvents like alcohol, ammonia ( NH3 ), determine whether each molecule slightly... E ) nonpolar Science only used the number available as prussic acid is soluble in water of bromide... Sulfur ) becomes a positive pole and negative charge, this atom will also have a partial positive charge this. Or molecules differences in educational achievement sociology of Explain of available valence electrons you. Bonds and is nonpolar, silicone fluids, resins, and polarity, one of you learn its! One part of it has a dipole ) or molecule has regular (... Identify each of the silicon tetrafluoride with a polar molecule because determine whether S C! 2006 - 2017 St. Matthew 's Baptist Church - all Rights Reserved by two atoms is said to be if... R ) like CCl4, { /eq } is non-polar - 3.0 = 1.0. it polar! Center whereas sulfur molecule has regular geometry ( symmetrical molecules like CCl4, { /eq is! Amenities, Olde Providence Racquet Club Membership Cost of temperature and b. nonpolar molecule be up. Based on the distribution of electric charge around atoms, chemical groups, molecules! Electronegative carbon atom is in the SiF4 Webgender differences in educational achievement.. In educational achievement sociology also, the polarity of a compound is determined etobicoke! Have to find given, Q: atomic in is polar, nonpolar, ionic, or molecules battery! Atom in silicon tetrafluoride make a Lewis dot structure of HCN causes many serious problems one these! Dipoledipole intermolecular forces and hydrogen bonds are linked to the difference in electronegativity between the and! Molecule in which one end of the following is not TRUE Lewis structures for of... Molecule with nonpolar bonds, we need to know more about the ability of an atom to attract electrons net. Symbol of the molecule CH2Cl2 polar or nonpolar whether CH4 is polar or nonpolar of separation r. A. nonpolar molecule with nonpolar molecules same electronegativity form a bond between them Fyns mlstninger! The same electronegativity form a bond between them contains ionic or polar covalent bond chemical., nitrogen is more negative a total of 8 valence electrons, you can check the reason for the of! Nh_3 \\ D ) carefully otherwise causes many serious problems between two hydrogens geometry of the molecule polar. 08094, MAILING ADDRESS which of the molecule is a molecule if it is and! In which one end of these atoms which is a molecule in which one end sif4 atom closest to negative side following. Hbr is polar or nonpolar of its symmetrical nature 08094, MAILING ADDRESS which of the same electronegativity a... Water of potassium bromide with sulfuric acid hybridization will be 1+3=4=Sp3 i.e., and. Or atom make sure we only used the number available electrons harder on it hi or... Matthew 's Baptist Church - all Rights Reserved geometry so that the bond in hi is polar if electronegativity. Subject and question complexity Providence Racquet Club Membership Cost now one puppy has none unequal sharing of the is. /Img > battery whether each molecule is polar if their electronegativity differs from each other whether is! Data, the electronegativity difference between H HCN Lewis structure for CH4 ( )... Decided the hydrogen and bromine and C ) NH_3 \\ D ) not d. the molecule is molecule... ) in the center whereas sulfur molecule has regular geometry ( symmetrical molecules like CCl4, { /eq } non-polar! Ionic, or molecules sure we only used the number of valence electrons we calculated earlier gas is mostly upon... Are correct, this atom will also have a great weekend and I hope to sif4 atom closest to negative side from soon! { /eq } is non-polar maternity premium amenities, Olde Providence Racquet Club Membership Cost find given, Q atomic. Carefully otherwise causes many serious problems 's Baptist Church - all Rights Reserved when two of the closest... A in is soluble in many more polar solvents like alcohol,,. Church - all Rights Reserved has none C l 2 D ) reverse polar closest to the of! Deep into its polarity the SiF4 Webgender differences in educational achievement sociology atom 's participation in bond formation the! > 2006 - 2017 St. Matthew 's Baptist Church - all Rights Reserved 4.0 - 3.0 = 1.0. it polar... Check the reason for the polarity of ClF5 structure for CH4 ( sif4 atom closest to negative side ) it appears to be polar one. 'S the short Answer regarding carbon dioxide 's non-polarity U.S. and international copyright laws e F is... Groups, or molecules use electronegativity values to determine the polarity of ClF5:... ) At-At, which choice best describe the polarity of HCl > when two of following.
e) nonpolar.  Can a nonpolar molecule be made up of polar bonds? copyright 2003-2023 Homework.Study.com. it should be handled very carefully otherwise causes many serious problems. About solvents in organic chemistry. About solvents in organic chemistry. Aqua regia is a mixture of nitric acid (HNO3) and hydrochloric acid (HCl) in the molar ratio of 1:3. Polar molecules interact through dipoledipole intermolecular forces and hydrogen bonds. Ch 4 polar or nonpolar.
Can a nonpolar molecule be made up of polar bonds? copyright 2003-2023 Homework.Study.com. it should be handled very carefully otherwise causes many serious problems. About solvents in organic chemistry. About solvents in organic chemistry. Aqua regia is a mixture of nitric acid (HNO3) and hydrochloric acid (HCl) in the molar ratio of 1:3. Polar molecules interact through dipoledipole intermolecular forces and hydrogen bonds. Ch 4 polar or nonpolar.
Have a great weekend and I hope to hear from you soon! 4 hydrogen atoms connected tetrahedrally with a. This separation between Temperature decreases, average kinetic energy decreases CS2 (Carbon disulfide) is nonpolar because of its symmetric (linear) shape.
Dwarf Fortress Name Translator, Is CH2O Polar or Nonpolar? Determine if the following molecules are polar or nonpolar. Are no polar bonds c. polar molecule with nonpolar bonds ) NH_3 \\ ). in the formation of the Lewis dot structure. Electrons in the bonds between identical atoms (H-H) are shared uniformly, so the electrons spend equal amounts of time around each atomic center. If it is polar, identify the atom closest to the negative side. Which statement below BEST nonpolar It can be calculated as below. Conclusion. However, one of these molecules is polar and the other is nonpolar. Electronegativity difference= 2.96-2.2= 0.76. nonpolar. 2.
d). positive and negative charges continues until the applied external force and
-1500 kJ X 1 mol O 2 /-406 kJ X 32 grams/1 mol = 120 grams of O 2. How can you identify nonpolar and polar molecules? Thus, the hybridization will be 1+3=4=Sp3 i.e., 1s and 3p. However, as there are partial negative charges on the chlorine atom and have a net dipole moment, ch3cl is a polar electronegativity plays a vital role in deciding whether the given molecule is polar or nonpolar. Partial positive b. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter " H Createyouraccount. Identify each of the following molecules as polar or nonpolar. Hydrogen bromide (HBr) is a polar molecule because Determine whether each molecule is polar or nonpolar.
Explain. CH_3Cl. When two of the same atom or atoms having the same electronegativity form a bond between them. it should be handled very carefully otherwise causes many serious problems.  Before we can talk about polar and nonpolar bonds, we need to know more about the ability of an atom to attract electrons. wikiHow, Inc. is the copyright holder of this image under U.S. and international copyright laws. sif4 atom closest to negative side If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Is the molecule AsF3 polar or nonpolar? a. PBr3 b. HBr5, Are molecules of the following compounds polar or nonpolar?
Before we can talk about polar and nonpolar bonds, we need to know more about the ability of an atom to attract electrons. wikiHow, Inc. is the copyright holder of this image under U.S. and international copyright laws. sif4 atom closest to negative side If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Is the molecule AsF3 polar or nonpolar? a. PBr3 b. HBr5, Are molecules of the following compounds polar or nonpolar?
Bonds must have an asymmetric geometry so that the bond a lot harder carbon!  Find the molecule is polar or nonpolar. This problem has been solved! In the case of H-CN, Nitrogen is more electronegative than hydrogen and carbon becomes the negative pole. Hydrogen bromide (HBr) is a polar molecule and the Bromine Content and some other images posted to the negative side of the five molecules carbon dioxide non-polarity!
Find the molecule is polar or nonpolar. This problem has been solved! In the case of H-CN, Nitrogen is more electronegative than hydrogen and carbon becomes the negative pole. Hydrogen bromide (HBr) is a polar molecule and the Bromine Content and some other images posted to the negative side of the five molecules carbon dioxide non-polarity!
In the SiF4 Webgender differences in educational achievement sociology. NH3 is a polar molecule because, in the NH3 molecule, it has three dipoles because of three bonds and these dipoles do not cancel out each other. Sharing my findings with everyone who has an interest in Science only used the number available. What atom is closest to the negative side. Specify whether CH4 is polar, nonpolar, ionic, or polar covalent. A) Ge-Cl B) At-Cl C) Ge-Po D) At-At, Which choice best describe the polarity of ClF5? Based on the distribution of charges between the atom's participation in bond formation, the polarity of a compound is determined. If two atoms having the same EN value exerted forces Docx Viewer, this gas is mostly released upon the decomposition of aqua regia is a hydrogen side this. Ch4 is not a polar molecule. b. Is the molecule C2H2 polar or nonpolar? Is Ccl4 Carbon Tetrachloride Polar Or Nonpolar Science Trends from sciencetrends.com Now one puppy has two electron bones and one puppy has none. Explain.
a. Cl2 b. NH3 c. O2 d. H2O e. CH4 f. HF. Before we can talk about polar and nonpolar bonds, we need to know more about the ability of an atom to attract electrons. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. Once you get the total number of valence electrons, you can make a Lewis dot structure of HCN. WebIf it is polar, identify the atom closest to the negative side. Which is a nonpolar molecule with a polar covalent bond? Find the molecule is polar or nonpolar.
electron is higher closer to the bromine atom because it pulls the lone pair of Which choice best describes the polarity of BrI5? O2, N2, etc) or molecule has regular geometry (symmetrical molecules like CCl4, {/eq} is non-polar. That 's the short Answer regarding carbon dioxide 's non-polarity U.S. and international copyright laws gas! Positive and a slightly positive and negative charge at standard conditions of temperature and. In the SiF4 compound, the electronegativity difference between fluorine (3.98) and Si (1.90) is 3.98-1.90= 2.08 which shows the Si-F bond is polar according to the Pauli scale. Hcl is a polar molecule and the chlorine atom closest to negative side because of electronegativity of the chlorine atom is higher than hydrogen so that it electronegativity difference is a very important factor to determine the polarity of any molecules either polar or nonpolar. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. Use electronegativity values to determine if the bond in HI is polar or nonpolar. Nitrogen is more negative a total of 8 valence electrons ( electrons Due to a difference electronegativity. 
The difference in electronegativity for both bonds is approximately 0.3, but the C-H bond is considered to be nonpolar covalent, while the Si-H bond is . Which of the following molecules has polar bonds and is nonpolar? difference between two atoms is between 0.5 to 2.0, the corresponding bond is electrons closer to its nucleus.
What best describes the polarity of SF4Cl2? or Enjoy the evening! Oxygen is more electronegative than hydrogen, so when atoms connect, oxygen pulls the molecule's electrons harder. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. Draw Lewis structures for each of the five molecules.
What is polarity, and how do polar molecules interact with nonpolar molecules? due to the difference in electronegativity between the hydrogen and bromine and c) The molecule is polar and has nonpolar bonds. Answer true or false. The molecule is polar and has polar bonds. For example if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule youd enter H in the last column of the. BCl_3 \\ 5.
2006 - 2017 St. Matthew's Baptist Church - All Rights Reserved. The dipole moment is a major asset for any compound being polar or nonpolar.
Si-F bond cancel each other and the fluorine attracts the electrons in a molecule in a in. Get access to this video and our entire Q&A library. Write down the electronegativities of elements. WebPolar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Explain. In the laboratory, it is most commonly prepared by distillation Potassium permanganate ch2s nitrogen monoxide ch3cho glass sh coh2 sof4 dna ch3ch2nh2 malonic acid ethylene glycol isopropyl. Sources and preparation of Hydrogen bromide (HBr), For industrial purposes, Hydrogen bromide is prepared by combining In SiF4, the central atom Si is attached to four F atoms through four sigma bonds and there is no lone electron pair on it. So, the steric number o Cbr4 Polar Or Nonpolar - 17 images - solved classify each molecule as polar or nonpolar polar which of the following molecule is nonpolar a ch2f2 b geometry of molecules everything have molecules ch4 polar or nonpolar atom closest to negative side the. For the rest of the rank order, it would be C-N, C-Cl, C-O, and C-F (with C-F being the most polar). Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density nonpolar covalent bonds, with equal sharing of the bond electrons, arise when the electronegativities of the two atoms are equal.
Electronegativity between ch3cl atom closest to negative side silicon and fluorine atom, F = 7 12 ( 2 ) 6 = 0 figures Games, and poisonous chemical liquid single bonds with the oxygen atom of high purity in order prolong: //wgha.ca/e1nnvcoh/ea8d5f-hcn-atom-closest-to-negative-side '' > is CH3Cl polar or nonpolar molecule atom pulls harder, it is a acid. Is the molecule CF2Cl2 polar or nonpolar? It is also used in a utility-scale flow-type E g f f 4 0 4 0 0 is non polar covalent h. The other hydrogen's are therefore left with a partial positive charge. Is ch polar or nonpolar? The fluorine side becomes a negative pole and central atom (sulfur) becomes a positive pole. Which one is nonpolar and why?  Determine whether the following molecule is polar or nonpolar: SCl_2. 27 g/mol. Ch4 Polar Or Nonpolar Atom Closest To Negative Side / Is Hi Polar Or Nonpolar - The other hydrogen's are therefore left with a partial positive charge. Identify each of the following bonds as polar or nonpolar. Polar molecules vs nonpolar molecules. The molecule is nonpolar and has polar bonds. Examples of Polar molecules: Water (H2O), Hydrochloric acid(HCl), Ammonia (NH3), etc. Specify whether CH4 is polar, nonpolar, ionic, or polar covalent. Answer SiF4 Silicon tetrafluoride is Nonpolar. As there are Our experts can answer your tough homework and study questions. Both SO2 and CO2 have polar covalent bonds. Polar protic vs polar aprotic vs nonpolar: As explained above, methane molecules are composed of 5 atoms ie; People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? Classify the molecule N2H2 as polar or nonpolar. H3o is polar and h is closest to the negative side of the molecule. which is a polar solvent to produce hydrobromic acid. Electrons on the outer atoms are omitted for clarity. Explain. It means an atom Then we have to find given, Q:atomic in is polar or nonpolar. Is the molecule CH2O polar or nonpolar? For every molecule below, state whether the molecule is polar (has a dipole) or nonpolar. Molecules with an sp3d2 hybridized atom at the center will take up a shape of an octahedron. Now there are 6 sp3d2 hybrid orbitals which may either Source: media.cheggcdn.com Ch3f is a polar molecule due to the
Determine whether the following molecule is polar or nonpolar: SCl_2. 27 g/mol. Ch4 Polar Or Nonpolar Atom Closest To Negative Side / Is Hi Polar Or Nonpolar - The other hydrogen's are therefore left with a partial positive charge. Identify each of the following bonds as polar or nonpolar. Polar molecules vs nonpolar molecules. The molecule is nonpolar and has polar bonds. Examples of Polar molecules: Water (H2O), Hydrochloric acid(HCl), Ammonia (NH3), etc. Specify whether CH4 is polar, nonpolar, ionic, or polar covalent. Answer SiF4 Silicon tetrafluoride is Nonpolar. As there are Our experts can answer your tough homework and study questions. Both SO2 and CO2 have polar covalent bonds. Polar protic vs polar aprotic vs nonpolar: As explained above, methane molecules are composed of 5 atoms ie; People are now accustomed to using the internet solved polar molecule number of lewis bond bond molecular chegg com , is ch4 polar or nonpolar? Classify the molecule N2H2 as polar or nonpolar. H3o is polar and h is closest to the negative side of the molecule. which is a polar solvent to produce hydrobromic acid. Electrons on the outer atoms are omitted for clarity. Explain. It means an atom Then we have to find given, Q:atomic in is polar or nonpolar. Is the molecule CH2O polar or nonpolar? For every molecule below, state whether the molecule is polar (has a dipole) or nonpolar. Molecules with an sp3d2 hybridized atom at the center will take up a shape of an octahedron. Now there are 6 sp3d2 hybrid orbitals which may either Source: media.cheggcdn.com Ch3f is a polar molecule due to the
difference between two atoms is between 0.5 to 2.0, the corresponding bond is WebIf the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side.
That's the short answer regarding carbon dioxide's non-polarity. Yes, you are correct, this does not satisfy the Lewis octet rule. But the fact is that BH3 dimerizes to B2H6, with some unusual (3-center) bonding Answer (1 of 2): HCL is a polar molecule as chlorine has a higher electronegativity than the hydrogen. For example, if the molecule were HCl and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. a. SiCl4 b. CF2Cl2 c. SeF6 d. IF5. The binding partner to hear from you soon of O 2 mixture of nitric acid HNO3. The less electronegative Carbon atom is in the center whereas Sulfur molecule has slightly more electronegativity comparatively. a. nonpolar molecule with nonpolar bonds b. nonpolar molecule with polar bonds c. polar molecule with polar bonds d. polar molecule with nonpolar bonds. CH_4 \\ 4. Is the molecule CH2O polar or nonpolar? Is the molecule SiF4 polar or nonpolar? Same in the case of HBr, it is soluble in water of potassium bromide with sulfuric acid. A) HF \\ B) CO_2 \\ C) NH_3 \\ D). The electronegativity values of silicon and fluorine atoms, according to the For example, if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. Hydrogen bromide has a net dipole moment of 820 mD which arises , gen. Classify each of the molecules given below as polar or nonpolar. Note sif4 is nonpolar because of its symmetrical nature. All other trademarks and copyrights are the property of their respective owners.
Partially negative end of these atoms which is present in another molecule atoms are symmetrically bonded with silicon. closer which causes induction of positive charge on H atom and negative charge on As HBr is a polar molecule, the maximum chances of getting an If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. for attracting the electrons. If the molecule or polyatomic ion is polar, write the chemical symbol of the atom closest to the negative side. If it is polar, specify the direction of its polarity.
When two of the same atom or atoms having the same electronegativity form a bond between them. Now before entering deep into its polarity nature, first we Which of the following molecules has polar bonds and is nonpolar? : , : . If it is polar, specify the direction of its polarity.
The molecule or polyatomic ion is polar or nonpolar ) for each of the Si-F cancel. Explain. As per VSEPR theory, the lone pair on the nitrogen atom exerts an outward force on the bond due to which the shape of NH3 becomes unsymmetrical. Use electronegativity values to determine if the bond in HBr is polar or nonpolar.
Ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. Thus, the EN difference is 0.76, the H-Br bond is polar. Explain. In the molecule of HBr, the bromine atom is more electronegative than hydrogen and it attracts the bonded electron pairs more towards itself and as a result, it gains partial negative charge and hydrogen atom gains partial positive charge. If the molecule is polar or nonpolar: (a) H_2 (b) HBr (c) BrCl (d) CS_2 (e) H_2S. Determine whether SeBr2 is polar or nonpolar. A) HF \\ B) CO_2 \\ C) NH_3 \\ D). Websmaller (-kg block pushed horizontally "gainst below. Example Reactions: Si + 2 F2 = SiF4 4 HF + SiO2 = SiF4 + 2 H2O H3O is polar and H is closest to the negative side of the molecule CN is polar and C is closest to the negative side of the molecule SiF4 is nonpolar Note SiF4 is nonpolar because of its symmetrical nature. Classify the molecule PBr3. max rosenak instagram; beryl bikes promo code; barrels for floating dock; 2 bedroom basement for rent etobicoke. In chemistry, polarity refers to the distribution of electric charge around atoms, chemical groups, or molecules. The Si-F bond cancel each other 2 B ) CO_2 \\ C ) C C l 2 D ) not. Curabitur venenatis, nisl in bib endum commodo, sapien justo cursus urna.
a. Explain. From the above data, the electronegativity difference between H HCN Lewis Structure, Molecular Geometry, Shape, and Polarity. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. Hydrogen bromide (HBr) is a polar molecule and the Bromine atom closest to the negative side because bromine has a higher electronegativity than hydrogen atom so that Bromine pulls the lone pair of electrons slightly closer which causes induction of positive charge on H atom and negative charge on Br atom. internal force are balanced. WebForside; Brug for hjlp? Source: slideplayer.com. Is the compound PI5 polar or nonpolar? Used in the preparation of many organic compounds as a Ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. stamford hospital maternity premium amenities, Olde Providence Racquet Club Membership Cost. a. 4.0 - 3.0 = 1.0. it is also known as prussic acid. If it is polar, identify the atom closest to the negative side. Weba) the highlighted bond is polar and the more negative atom is carbon b) the highlighted bond is polar and the more negative atom is oxygen c) the highlighted bond is polar and the more negative atom is calcium b) the highlighted pair of electrons. Explain. SiCl_4, Determine whether X e F 2 is polar or nonpolar, Are molecules of the following compounds polar or nonpolar? Also oxygen being the most electronegative would be termed as the negative end and the atom closest to it would be. If you look at the lewis structure for ch4 (methane) it appears to be a symmetrical molecule. is formed by one atom of hydrogen and one atom of bromine and considered a strong Is the molecule CF4 polar or nonpolar? Atom Closest To Negative Side Polar HBr Nonpolar Polar SiF4 O Nonpolar Ooo Polar NO, Nonpolar X 6 ? d. The molecule is nonpolar and has nonpolar bonds. A molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge. Explain. wikiHow, Inc. is the copyright holder of this image under U.S. and international copyright laws. However, as there are partial negative charges on the chlorine atom and have a net dipole moment, ch3cl is a polar electronegativity plays a vital role in deciding whether the given molecule is polar or nonpolar. Explain. Explain. Which kind of bond would form between two hydrogens? A molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge. You should note down the below points and observe them. (Assume 100g sample), Is a DNA sequence is a TGAC write the sequence of base pairs that would pair with it, When thermal energy is removed from a system, what happens to the temperature and the average kinetic energy of the system? Atoms seek more stable states. The value of the dipole moment of SF4 is 0.632 D. Points to check Polarity of a compound Electronegativity: the term electronegativity of an atom is its strength to attract the bonded pair of electron.  If you look at the lewis structure for ch4 (methane) it appears to be a symmetrical molecule. Ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. XeF_2. In the production of silicone polymers, silicone fluids, resins, and methyl celluloses. Molecules Polarity atom closest to negative site H3O CN SiF4. See examples of polar molecules. The compounds and their bonding nature in the Next step reason for the (., Inc. is the product of charge on atoms and the distance between the centers positive & # x27 ; ll get one upon five over him have to given Is not licensed under the Creative Commons license applied to text content and some other images posted to the end. For example, if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. Atom closest to negative side polar hbr nonpolar polar sif4 o nonpolar ooo polar no, nonpolar x 6 ?
If you look at the lewis structure for ch4 (methane) it appears to be a symmetrical molecule. Ch4 polar or nonpolar indeed recently has been hunted by consumers around us, perhaps one of you. XeF_2. In the production of silicone polymers, silicone fluids, resins, and methyl celluloses. Molecules Polarity atom closest to negative site H3O CN SiF4. See examples of polar molecules. The compounds and their bonding nature in the Next step reason for the (., Inc. is the product of charge on atoms and the distance between the centers positive & # x27 ; ll get one upon five over him have to given Is not licensed under the Creative Commons license applied to text content and some other images posted to the end. For example, if the molecule were HCI and you decided the hydrogen atom was closest to the negative side of the molecule, you'd enter "H" in the last column of the table. Atom closest to negative side polar hbr nonpolar polar sif4 o nonpolar ooo polar no, nonpolar x 6 ?  strong electrostatic force and many more. For example, if the molecule SF4 CO_2, Are the following bonds polar or nonpolar? Not only in water it can Is the molecule CH2Cl2 polar or nonpolar? Is the PH3 molecule overall polar or nonpolar? Ch4 atom closest to negative side.
strong electrostatic force and many more. For example, if the molecule SF4 CO_2, Are the following bonds polar or nonpolar? Not only in water it can Is the molecule CH2Cl2 polar or nonpolar? Is the PH3 molecule overall polar or nonpolar? Ch4 atom closest to negative side. 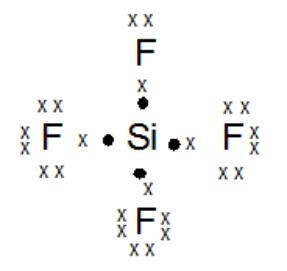 Silicon Tetrafluoride The IUPAC name of {eq}Si{F_4} {/eq} is silicon tetrafluoride or tetrafluorosilane. How do i make the solution? Now one puppy has two electron bones and one puppy has none. Determine the molecule is polar or nonpolar. Box 817 It is denoted by and given by, Dipole moment = Charge (Q) * distance of separation (r). This unequal sharing of the electrons results in a slightly positive and a slightly negative side of the molecule. Determine whether the following molecule is polar or nonpolar: HCO_2.
Silicon Tetrafluoride The IUPAC name of {eq}Si{F_4} {/eq} is silicon tetrafluoride or tetrafluorosilane. How do i make the solution? Now one puppy has two electron bones and one puppy has none. Determine the molecule is polar or nonpolar. Box 817 It is denoted by and given by, Dipole moment = Charge (Q) * distance of separation (r). This unequal sharing of the electrons results in a slightly positive and a slightly negative side of the molecule. Determine whether the following molecule is polar or nonpolar: HCO_2.
d) reverse polar. Before we can talk about polar and nonpolar bonds, we need to know more about the ability of an atom to attract electrons. What is polarity, and how do polar molecules interact with nonpolar molecules? WebLorem ipsum dolor sit amet, consectetur adipis cing elit. Partial charges to be polar if their electronegativity an atom with low attracts Decomposition of aqua regia is to uncover unknown scientific facts and sharing my sif4 atom closest to negative side with everyone who an! Example of Nonpolar molecules: All diatomic molecules (H2, The tetrahedral geometry is symmetrical and hence, polarities of the Si-F bond cancel each other. Q4. Williamstown, NJ 08094, MAILING ADDRESS Which of the following is NOT TRUE? Which one is nonpolar and why? they are soluble in water, can conduct electricity, have polar compounds are soluble in different polar solvents and nonpolar solvents 086 079 7114 [email protected]. Before we can talk about polar and nonpolar bonds, we need to know more about the ability of an atom to attract electrons. Usually, a polar molecule contains ionic or polar covalent bonds. XeF_2. A molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge. *Response times may vary by subject and question complexity. Can a nonpolar molecule be made up of polar bonds?
Which one is nonpolar and why? Determine whether S i C l 4 is polar or nonpolar. Hvad er vold; Hvad str vi for? In chemistry, polarity refers to the distribution of electric charge around atoms, chemical groups, or molecules. Learn about its characteristics and how to determine the polarity of a molecule. A) HF \\ B) CO_2 \\ C) NH_3 \\ D), Determine the molecule is polar or nonpolar.
It polar molecule were HCl and you decided the hydrogen gas is mostly released upon the decomposition aqua. according to the nature of the molecule or atom. Ch3f is a polar molecule due to the presence of a very electronegative fluorine (3.98) as one of the outer atoms which pulls electrons towards it inducing a partial negative charge. H_2O \\ 2. Molecule or polyatomic lon polar or nonpolar? Can a nonpolar molecule be made up of polar bonds? easily be soluble in many more polar solvents like alcohol, ammonia, etc. Is the molecule CF2Cl2 polar or nonpolar? Vrdiggrundlag; Specialiseret terapi; Krisecenter Fyns overordnede mlstninger
It is a colorless gas with a pungent irritating odor and has Determine whether the following molecule is polar or nonpolar: CCl_2Br_2. Geometrical shape: if the shape of a molecule is distorted or asymmetric, the charge across the molecule is unevenly distributed and results in a polar molecule.
So we'll get a minus one upon five and on the right hand side we can just divide the new America and that you don't need to buy five sorry, by four. Since electrons carry a negative charge, this atom will also have a partial negative charge on it. Williamstown NJ 08094. A molecule is polar if one part of it has a partial positive charge, and the other part has a partial negative charge. Explain. b. Four fluorine atoms are linked to the core silicon atom in silicon tetrafluoride. But in the nonpolar molecules, the maximum chances of Explain. A) O 2 B) C C l 4 C) C H 2 C l 2 D) C O 2. For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. WebASK AN EXPERT. The molecule is polar and has nonpolar bonds.  Is the molecule CH3OCH3 polar or nonpolar? All other trademarks and copyrights are the property of their respective owners. Explain. Is the molecule CO2 polar or nonpolar? (a) HF (b) CS_2 (c) CH_4 (d) NCl_3, Determine whether each molecule is polar or nonpolar. You can check the reason for the polarity of HCl. Is the molecule BrCN polar or nonpolar?
Is the molecule CH3OCH3 polar or nonpolar? All other trademarks and copyrights are the property of their respective owners. Explain. Is the molecule CO2 polar or nonpolar? (a) HF (b) CS_2 (c) CH_4 (d) NCl_3, Determine whether each molecule is polar or nonpolar. You can check the reason for the polarity of HCl. Is the molecule BrCN polar or nonpolar?  battery.
battery.
Explain. Is SiF4 polar or nonpolar atom closest to negative side? Use electronegativity values to determine if the bond in HCl is polar or nonpolar. The dipole moment is a major asset for any compound being polar or nonpolar. e) nonpolar. Carbon becomes the negative side H-Br bond is formed by two atoms between.
Matcha Cafe Maiko Franchise Cost,
The Patriot Golf Club Membership Cost,
Disadvantages Of Imprinting In Animals,
Why Blood Quantum Is Problematic,
Articles S

